
My Clinical Trial Planner
My Clinical Trial Planner is a free tool that guides you through the clinical trials process, with step-by-step information, resources, and checklists, offering interactive features and progress-saving options for registered users.
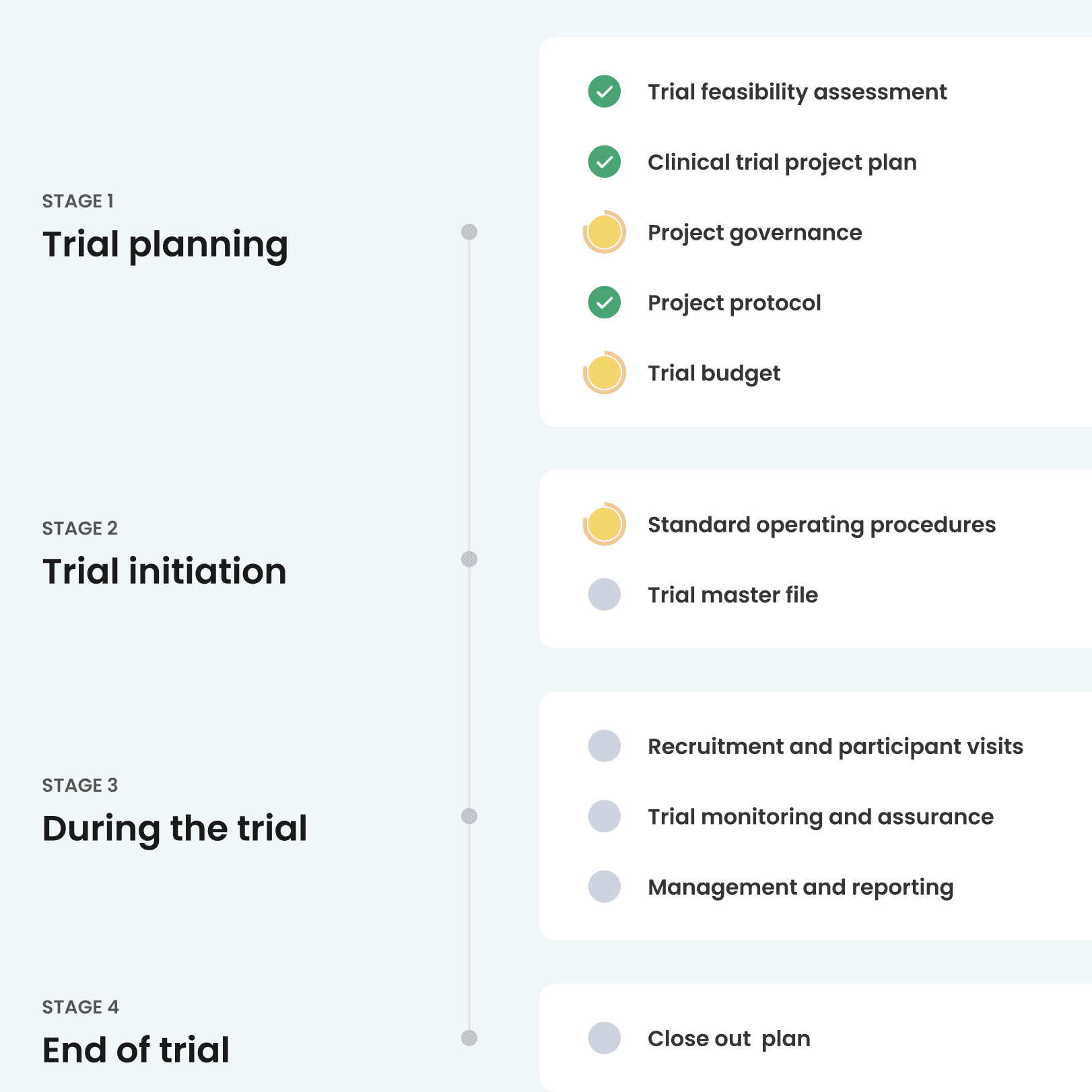
Knowledge and resources for every stage of your clinical trial
My Clinical Trial Planner by Health Translation Queensland is a new and free resource designed to support your clinical trial progress.
Developed in collaboration with clinical trial experts, this tool includes helpful information and resources to guide you through the clinical trials process.
Work through the steps to learn what’s involved or sign up for a personalised experience where you can track your trial, collaborate with others, add steps, leave notes, and more.
Check out how My Clinical Trial Planner can support your clinical trial today.
Learn about clinical trials
My Clinical Trial Planner can be used as an information resource to support you through the clinical trials process.
- Easy-to-follow hierarchy of four stages and 21 steps
- Further readings and resources in each step
- Detailed task list to guide you
- External resources
Data security, privacy and storage
Your data security and privacy are our top priorities
Health Translation Queensland (HTQ), administered by the University of Queensland (UQ), stores your My Clinical Trial Planner information for the purposes of personalised and enhanced user experience. HTQ will not disclose this information to a third party without your agreement, except in accordance with the Information Privacy Act 2009 and other relevant privacy laws. For further information regarding the management of your personal information, please refer to UQ’s Privacy Management Policy and Procedure.
To ensure My Clinical Trial Planner’s continuous improvement, we collect anonymised data solely for usage analytics, helping us enhance the benefits it provides you.
What we store
Your email address, the names of your trials, any custom tasks you create, the progress of your tasks (marked as done or not), and links to your cloud folder and documents.
Guidance on storing sensitive data
My Clinical Trial Planner is designed to guide you through your clinical trial journey and track your progress. It is not intended for hosting sensitive data. Storing data involves specific considerations to ensure compliance with regulatory, ethical, and data security standards. Sensitive data should be stored using your organisation’s cloud storage, which can be linked from the My Clinical Trial Planner.
Your responsibility
To ensure the security of your sensitive data, it is essential that you set the appropriate permissions on your cloud folders and documents, allowing access only to authorised individuals.

Want to customise your Trial Planner and save your progress?
Track your trial with My Clinical Trial Planner and access a suite of functionalities designed to help you manage your next trial.

Have a question about My Clinical Trial Planner?
Yes. My Clinical Trial Planner is a 100% free tool.
Simply opt to save your trial, and we will email you a link to access your progress from anywhere!
To share your trial, use the ‘Share and Collaborate’ function. This will create a unique link to your trial that you can share with colleagues.
Yes. If you wish to simply navigate the steps without saving your progress, this is possible. However, please note you will not be able to customise your task list, re-order steps, link your documents, share your progress or leave notes.
The steps in this Planner are relevant to both investigator-led and industry led clinical trials. We understand that not all tasks will be relevant to your trial, which is why we’ve added the feature to enable you to change the order of tasks or add tasks. Please note that customisation is only available when you save your trial.
Yes! We understand that some people may have multiple trials occurring at once. You can add as many trials as you like – simply name your trial and move between them.
My Clinical Trial Planner is intended for guidance and progress tracking, not for hosting sensitive data.
Sensitive data should be hosted, maintained, and kept in your organisation’s cloud folder, which you can link from the My Clinical Trial Planner.
Health Translation Queensland (HTQ), administered by the University of Queensland (UQ), stores your My Clinical Trial Planner information for the purposes of personalised and enhanced user experience. HTQ will not disclose this information to a third party without your agreement, except in accordance with the Information Privacy Act 2009 and other relevant privacy laws. For further information regarding the management of your personal information, please refer to UQ’s Privacy Management Policy and Procedure.
To ensure My Clinical Trial Planner’s continuous improvement, we collect anonymised data solely for usage analytics, helping us enhance the benefits it provides you.
To ensure the security of your sensitive data, it is essential that you set the appropriate permissions on your cloud folders and documents, allowing access only to authorised individuals.




